There are many chemicals in seawater that make it salty. Most of them get there from rivers carrying chemicals dissolved out of rock and soil. The main one is sodium chloride, often just called salt. Most seawater has about 35 g (7 teaspoons) of salt in every 1,000 g (about a litre) of water. This doesn’t sound very much, but it would take close to two 6 m shipping containers full of salt to make an Olympic-size swimming pool as salty as the sea.
The commonest way to record salinity is to measure the amount of salt in 1,000 g of water, so it is referred to as ‘parts per thousand’ or ppt. Most of the ocean has a salinity of between 34 ppt and 36 ppt.
Some properties of water are changed by having salt in it:
- Salt makes seawater more dense than freshwater.
- Salty water needs to be colder than freshwater before it freezes.
Variation in salinity
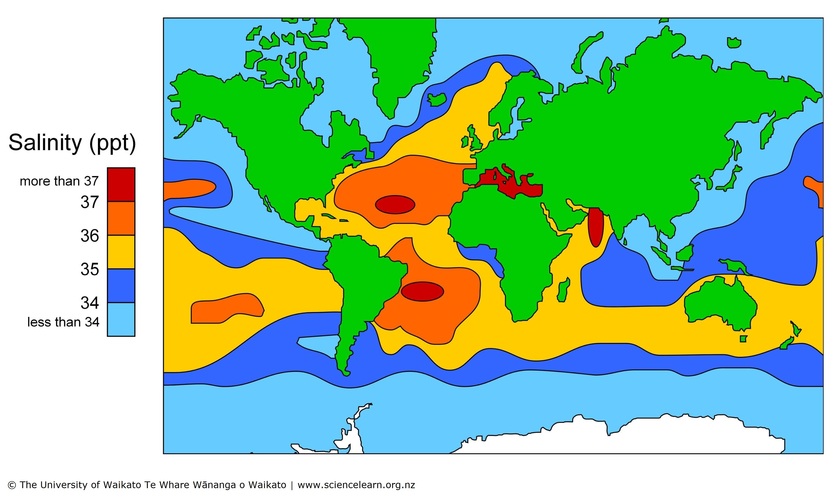
The salinity of the ocean varies from place to place, especially at the surface. Much of the ocean has salinity between 34 ppt and 36 ppt, but there are places that tend to be higher or lower.
Places of higher salinity
There are parts of the ocean where hardly any rain falls but warm dry winds cause lots of evaporation. This evaporation removes water – when water vapour rises into the atmosphere, it leaves the salt behind, so the salinity of the seawater increases. This causes the seawater to become denser. You can see on the map that the north and south Atlantic have high salinity – these are areas where there are strong winds and not much rain.
The Mediterranean Sea in Europe has very high salinity – 38 ppt or more. It is almost closed from the main ocean, and there is more evaporation than there is rain or extra freshwater added from rivers.
Places of lower salinity
Some parts of the ocean have lots of rain. The freshwater added at the surface dilutes the seawater, reduces the salinity and so makes the seawater less dense. Seawater can also be less saline near land, where rivers add freshwater.
The ocean around Antarctica has a low salinity of just below 34 ppt, and around the Arctic it is down to 30 ppt in places. Thawing icebergs add freshwater – icebergs that have broken off ice sheets formed over land do not contain salt, and the freezing of seawater into ice floes removes more salt.
The Baltic Sea, almost enclosed by northern Europe and Scandinavia, has a very low salinity of about 10 ppt. This is mainly due to the huge amount of freshwater added from hundreds of rivers.
What happens when salinity changes
The difference between 34 ppt and 36 ppt salinity doesn’t sound very much, but it is enough to cause a difference in density. Even slightly denser seawater sinks below less dense water.
However, the effect is greater if the salty water gets cold, as temperature has a greater effect on density than salinity does. A combination of high salinity and low temperature makes seawater so dense that it sinks to the bottom of the ocean and flows across ocean basins as deep, slow currents.
Related content
The ocean is a system of chemical, physical and biological processes. Some of these processes are driven by basic science concepts – temperature, ocean density and salinity (saltiness) – that help cause currents and affect climate. Find out more about ocean motion.
Activity ideas
Try these hands-on student activities with your class – there are centered around the properties of seawater, density and Saline currents.