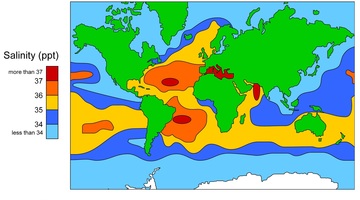
Water density changes with temperature and salinity. When water freezes at 0°C, a rigid open lattice (like a web) of hydrogen-bonded molecules is formed. It is this open structure that makes ice less dense than liquid water. This is why icebergs float.