Dr Kim Currie from NIWA studies carbon dioxide in the upper ocean around New Zealand. This helps her understand the role of the ocean in the carbon cycle and how the ocean and the atmosphere interact.
Measuring ocean chemistry
Every two months from 1998 to 2008, NIWA scientist Dr Kim Currie went to sea in the research vessels Munida or Polaris. Along the same 60 km line off the east coast of the South Island, she measured temperature pH and carbon dioxide of water near the surface. Kim had to build her own instrument for measuring carbon dioxide as there was not one accurate enough available. These measurements have given Kim a picture of changes from season to season and from year to year.
Rising carbon dioxide
The ocean takes up carbon dioxide from the atmosphere as part of the natural carbon cycle. The amount of carbon dioxide in the atmosphere is rising. This suggests that the ocean is not keeping up with extra gas added to the atmosphere, which comes mainly from human industrial activities.
Kim wants to know how the seas around New Zealand are involved in the increase in carbon dioxide in the atmosphere – has carbon dioxide in the water increased too? Her research is helping her and others to understand the carbon cycle and how the ocean and atmosphere systems work together.
Over the 10 years of Kim’s study she found:
- not surprisingly, the surface water was warmer in summer and cooler in winter – over the length of the study, surface temperature did not rise overall
- carbon dioxide in the surface water was higher in winter than in summer – over the length of the study, it increased very slightly overall
- pH went down very slightly
- carbon dioxide in the atmosphere of New Zealand increased (measured in a separate study).
Cold water holds more carbon dioxide than warm water, which partly explains the higher carbon dioxide levels in winter. With carbon dioxide in the atmosphere increasing, it would be expected that the level in the ocean would rise too. This didn’t really show much in her study, and Kim says the answer lies partly in living organisms in the ocean.
Phytoplankton (tiny drifting marine plants) near the surface use up carbon dioxide during photosynthesis, so carbon dioxide moves from the atmosphere to replace it. When organisms die, many sink into deep water, taking much of the carbon with them. Carbon dioxide entering the upper ocean is also chemically converted into carbonic acid and bicarbonate ions, and biologically converted into organic material.
Acidification
Some of the carbon dioxide that enters seawater forms carbonic acid. An increase in carbon dioxide entering the ocean makes the ocean more acidic – this shows in the decrease of pH. StatsNZ notes that ocean aciditiy has increased 7.1% in New Zealand's subantarctic surface waters between 1998-2017. Increased acidification can cause the shells of some organisms to dissolve and also make it harder for the organisms to make shells in the first place. Associate Professor Abby Smith is investigating the impact of ocean acidification on bryosoans. Other marine species from plankton to crustaceans to molluscs are particularly at risk.
Expanding the study
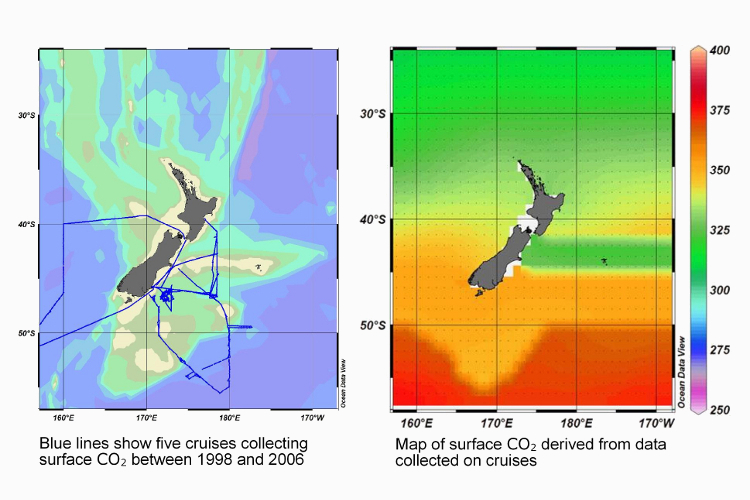
Taking ships to sea for research is expensive, so Kim was keen to find an alternative. A number of cruises around New Zealand provided Kim with some carbon dioxide data but only along the paths the vessels travelled. This data has been extrapolated to cover a wider area, but it might not be very accurate. However, data from these cruises has let Kim develop a method for determining carbon dioxide in surface water from parameters that can be measured from space, such as sea-surface temperature and chlorophyll concentration. Hopefully, satellites will prove a much cheaper source of information in the near future, and allow greater coverage.
Nature of science
Kim’s work highlights the need for careful experimental design. Her 10-year study gave her data from only a small area but over a long enough period to pick up seasonal and yearly variation. Cruises gave data over a much wider area, but only at a single point in time. Satellites are a way to provide continuous data over wide areas.
Related content
Dr Kim Currie studies the role of the ocean in the carbon cycle – the movement of the element carbon around the Earth. She’s particularly interested in the exchange of carbon dioxide between the ocean and the atmosphere. Find out more about her research into carbon dioxide in the atmosphere.
Activity idea
In this activity, students observe how chicken eggs can be used to simulate the potential effects of increasing ocean acidity on marine animals with calcium carbonate shells or skeletons, for example, bryozoans and cockles.
Useful link
Running since 1998, the Munida time-series, led by Kim has been collecting ocean chemistry measurements along a 65 km line off the Dunedin coast every two months, find out more in this 2022 Radio NZ Our Changing World programme When good science takes time.
Kim was involved in setting up The New Zealand Ocean Acidification Observing Network (NZOA-ON) project – a nationwide network of stations to monitor coastal pH.
Find out more about NIWA's work investigating ocean acidification.
See the StatsNZ website for information, resources and data on ocean acidification.