Matter is anything that has mass and occupies space. A useful way to start thinking about matter is to think about the different materials, or substances, that it can be made into.
These materials make up the objects around us, and each of these materials has different properties or characteristics that can be observed or tested. Scientists, technologists and engineers investigate these materials – they experiment with them, compare their properties and relate the results to possible uses.
Types of materials
There are many different types of materials. Some examples of everyday materials are plastics, metals, fabric and glass.
Find out more about plastic products in the article Plastics and recycling.
Find out more about metals and what happens when they mix in the article Metals, alloys and metal compounds.
Ceramic materials are used to make traditional pottery, right through to advanced ceramics used in engineering and medicine. These inventions require scientists to understand the properties of minerals. You can learn more in the article What are minerals?
Wool is another traditional material that has undergone innovation. Investigate the properties of wool and how they link to its uses in the student activity Exploring wool fibre properties.
Some other fascinating, less well known materials include nanofibres, biological materials and composites.
Examples of properties
When we refer to the properties of a material, we are talking about features we can sense, measure or test. For example, if we have a sample of metal in front of us, we can identify that this material is grey, hard and shiny. Testing shows that this material is able to conduct heat and electricity and that it will react with an acid. These are some of the metal’s properties.
It is important to decide if you are investigating the properties of a material or of an object. For example, are you identifying the properties of a spoon (an object), or are you looking at properties of the material it is made of, for example, stainless steel? Properties like shape and mass may be different for different objects, even when they are made of the same material. Density is a useful property for making comparisons between different materials.
Use this activity to learn more about density.
Other properties of materials can include their viscosity and conductivity.
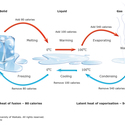
A commonly talked about property is the state or phase of matter. There are currently five different states of matter that have been identified: solids, liquids, gases, plasma and Bose-Einstein condensate. The last two of these are much less well known.
It is important to note that the state of matter refers to the positioning and movement of the particles that make up a material and not the material itself.
You can learn more about states of matter in the article States of matter.
Physical versus chemical
Sometimes it can be useful to distinguish between different types of properties. Physical properties refer to properties that can be observed or measured without changing the composition of the material. Examples include colour, hardness and smell and freezing, melting and boiling points.
Chemical properties are discovered by observing chemical reactions. They include combustion point, reactivity with acids and toxicity.
Changing material properties
Processes such as mixing, heating and cooling can change materials and their properties. This can be useful as the new properties may be better suited for particular purposes. For example, mixing certain metals can create a material that is both strong and lightweight.
Related content
Wanting to explore more chemistry ideas and chemistry in a variety of different contexts? Take a look at the wide range of content we have on the Hub, including the properties of matter and atoms and molecules concepts.
Check out our related resources about mixtures, recycling, melting and fire, or visit our context-based chemistry articles looking at elements, limestone, plasma and digestion chemistry.
We also have two recorded teacher PLD webinars: Chemistry made simple – properties of matter and Chemistry made simple –atoms.