A major risk of pig cell transplants is the spread of disease from pigs to humans. At Living Cell Technologies (LCT), they test donor pigs, pig cells and transplant recipients for pathogens such as viruses and bacteria.
Pig cell transplants and viruses
Pig cell transplants are a type of xenotransplant. A major risk of xenotransplantation is the transmission of known (or unknown) diseases from one species to another.
LCT uses several strategies to minimise the risk of disease transmission from pig to humans, such as:
- pig cells are from designated pathogen-free pigs
- pig cells are encapsulated
- testing for pathogens.
Pig cells are from designated pathogen-free pigs
LCT uses a unique breed of disease-free pigs as the source of pig cells. These pigs are kept in a pathogen-free facility and tested regularly to ensure they aren’t carrying any diseases. The article Designated pathogen-free pigs – origins and welfare has further information.
Pig cells are encapsulated
LCT’s pig cell transplants are encapsulated to protect them from being rejected by the recipient’s immune system. This also means the recipient doesn’t need to take toxic immune-suppressing drugs that make them more vulnerable to infections. Find out more on Preventing pig cell transplant rejection in this article.
Testing for pathogens
LCT has a world-class molecular diagnostics lab that can test for many different pathogens. They test the donor pig herd, pig cells, pig cell transplant recipients and their close contacts.
Blood and faecal samples from the pigs are taken every 4 months and analysed in the lab for about 30 different disease-causing pathogens, including hepatitis E, swine flu, circovirus, porcine herpesvirus and porcine endogenous retrovirus. Encapsulated pig cells are tested before transplanting, and transplant recipients are tested for up to 2 years after they’ve received a transplant.
Techniques for pathogen testing
The lab at LCT uses polymerase chain reaction (PCR) to test for the presence of viral DNA in samples. LCT uses a new form of PCR that can copy, detect and quantify viral DNA. It’s a fast, sensitive and specific method that can be used to detect specific viruses.
Serology is the testing of samples for the presence of antibodies to viruses that would indicate a viral infection. At LCT, they use standard serology methods to measure antibodies, such as enzyme-linked immunosorbent assays (ELISA) and latex agglutination tests (LAT).
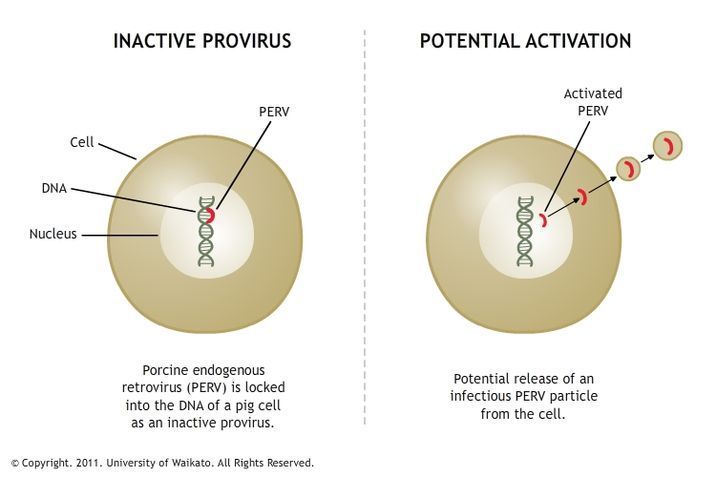
PERV: a pig retrovirus
Porcine endogenous retrovirus (PERV) is found in all pigs’ DNA. PERV has two forms – a harmless provirus, which is locked into the pigs’ DNA, and a viral particle that could potentially infect other species.
Is PERV a risk for pig cell transplant recipients?
The ban on pig to human transplants has now been lifted in some countries. In New Zealand, for example, LCT’s pig cell transplant research is proceeding under rigorous controls. LCT sources its cells from designated pathogen-free pigs while they have copies of the provirus in their DNA, but it has never been shown to release a viral particle.
A study published in 2016 reported no transmission of PERV during a DIABECELL clinical trial in Argentina.
Responding quickly to virus outbreaks
It is important that LCT can respond quickly to new virus outbreaks and test their pigs. For example, the H1N1 flu virus epidemic started in April 2009. It was initially called swine flu because of the similarity of the virus to pig flu viruses, but it spread from human to human, not from pig to human. Once LCT had tested and confirmed their pig herd was free of the virus, they were able to continue their research.
Find out more about virus strains and the H1N1 flu virus in this article.
Useful links
Keep up to date with the latest developments with Algorae Pharmaceuticals Ltd (previously LCT).