Temperature is a measure of the average energy of the particles that make up a substance. It relates to the idea of hotness and coldness. If an object feels hotter, generally it has the higher temperature.
Heat is a measure of the total amount of thermal energy in a body. Although a cup of boiling water and a full kettle of boiling water both have the same temperature (100°C), the amount of heat or thermal energy present in the water of the full kettle is far greater then the water in the cup.
Heat flows from a hotter object to a colder one. If no net heat flow occurs between two objects, the objects have the same temperature.
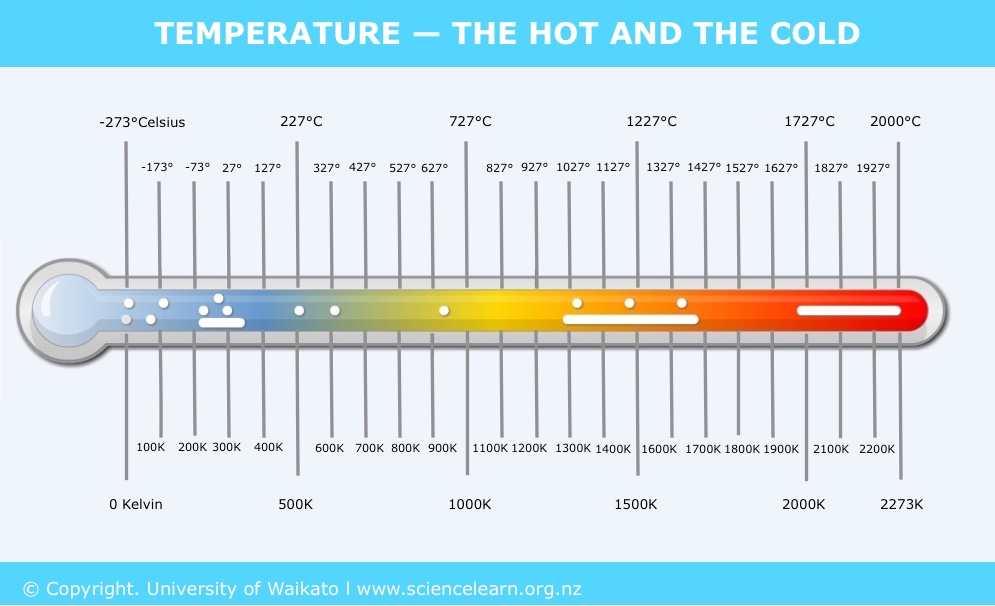
Temperature scales – Celsius, Fahrenheit and Kelvin
Three temperature scales are in general use worldwide today. In scientific reports, only two of them are used – Celsius and Kelvin.
Celsius, also known as centigrade, is a temperature scale that is named after the Swedish astronomer Anders Celsius (1701–1744). 0°C is defined as the freezing point of water and 100°C as the boiling point of water, both at standard atmospheric pressure.
The Fahrenheit scale is named after the 18th century German physicist Daniel Gabriel Fahrenheit. The freezing point of water is defined as 32°F and the boiling point of water as 212°F.
The Kelvin scale is a temperature scale based on a theoretical point at which all thermal energy in any material is reduced to 0. This point is known as absolute zero and is defined as zero kelvin (0 K). On the Celsius scale, it is -273.15°C.
The Kelvin scale and the kelvin are named after the British physicist and engineer William Thomson, 1st Baron Kelvin (1824–1907).
A kelvin unit has the same size as the Celsius degree: 1 K = 1°C. Absolute zero is zero kelvin (0 K) or -273.15°C, and 0°C is 273.15 K.
In scientific formulae, articles and reports, temperature is frequently given in the unit kelvin. The symbol ‘T’ is often used in scientific formulae to represent temperature in kelvin.
Low temperature – superconductivity
Superconductors are materials that lose all resistance to electric current when cooled to a certain temperature. This temperature – the critical temperature – depends on the structure and composition of the material.
The advantage is that larger electric currents can be carried through thinner wire, with minimal energy losses.
At the present time, liquid helium is the coolant most often used in superconductor technologies.
Helium exists in liquid form only at extremely low temperatures. The boiling point of helium-4 is -269°C or 4 K at standard atmospheric pressure.
Dutch physicist Heike Kamerlingh Onnes first liquefied helium-4 in 1908. By using liquid helium as a coolant, he discovered superconductivity.
Researchers at Industrial Research Limited (IRL) in Wellington, New Zealand, have developed a ceramic material that becomes superconductive when cooled with liquid nitrogen (-196°C or 77 K). The critical temperature of the ceramic is high compared with that of the alloys found in high-field magnets that are part of hospital MRI scanners. Superconducting magnets that make use of this technological advance are far cheaper to run.
Liquid helium and nitrogen compared
If liquid helium is cooled to -271°C or 2 K, it becomes a superfluid. In this state, it can flow freely, even upwards, with little apparent friction .
| | |
High temperature – advanced ceramics
The use of temperature in firing ceramics is critical to the hardening process. Traditional clay-based ceramics are normally heated in a kiln to temperatures within the range 1,100–1,300°C.
With some of the new high-performance ceramics, temperatures up to 1,800°C are needed. Specially designed furnaces have been developed to meet this need.
In the production of advanced ceramics, finely milled powders are shaped into a green body, and this is then heated to a very high temperature that allows the fine particles to fuse at their edges. This process is known as sintering.
Advanced ceramics have an amazing range of properties and uses. They can be designed and engineered to solve just about any problem or challenge we face.
Nature of science
Science research is often driven by identification of potential technological applications. The development of a ceramic material that conducts at a higher temperature has resulted in a switch from costly liquid helium as a coolant to cheaper liquid nitrogen.
Activity ideas
- Investigating temperature – view the interactive Temperature – the hot and the cold and participate in a class discussion.
- Liquid nitrogen demonstrations – observe a teacher demonstrating changes in the properties of common substances when cooled with liquid nitrogen.
- Investigating heat absorption – students use thermometers inside water-filled soda bottles to investigate how dark and light colours affect heat absorption.